Gene studies advance medicines and profit for Alzheimer’s
Denise Combs remembers the moment when she first realized her husband John might be suffering from memory loss.
“He was a real estate broker for 38 years and always wore a necktie, but one Easter morning… he couldn’t knot his tie. He had done [it] all his adult life,” she says. The incident, combined with a gradual difficulty in speech and an increasingly frequent knack for losing things, caused Combs to begin to search for a doctor.
She made an appointment for her husband with Dr. James Mastrianni, director of the Center for Comprehensive Care and Research on Memory Disorders at the University of Chicago Medical Center, a specialist in Alzheimer’s disease and similar disorders.
“It’s a two-hour drive from Rockford where we live, but when you choose a doctor for [a] specialty, [you’ll] go to the ends of the earth to see [him or her],” Combs says. In 2007, after a thorough physical exam and neuropsychological tests conducted by several physicians that lasted more than six hours, John Combs was diagnosed with frontal temporal dementia. Mastrianni prescribed Aricept, a drug primarily given to Alzheimer’s patients.
“These are cholinesterase inhibitors that block an enzyme in the brain that naturally breaks down one of the neurochemicals involved in formation of memories. The goal is to build up that neurochemical,” Mastrianni says. “This is not the root cause of the disease. These medicines simply mask some of the features of the disease, but they don’t stop the disease or inhibit its progression.”
“People ask me whether it’s working, but I really can’t tell, because I don’t know what my husband would be like without it,” Combs says.
Researchers are currently making major advances in the detection of diseases such as Alzheimer’s so that medications can be developed to stop their progression before it has created irreparable damage to brain cells.
As one of 44 universities and research institutions in the United States that participated in a study to identify the causes of the disease, the Cognitive Neurology and Alzheimer’s Disease Center at Northwestern University’s Feinberg School of Medicine contributed DNA samples from Alzheimer’s patients to the research project. This large collaborative effort resulted in identifying four new genes linked to the disease. According to Dr. Marsel Mesulam, director of the center, the study was based on samples from over 8,000 patients with late-onset Alzheimer’s disease and more than 7,000 people in the control group.
“As a result of this large group, we were able to find four genes that were associated with increased risk of developing late-onset Alzheimer’s disease,” he explains. “There are six other known risk genes, so now researchers have to try to figure out what they do, and then to see if manipulating these gene products in some pharmacological way will help to decrease the risk of developing Alzheimer’s disease, therefore eventually preventing it.”
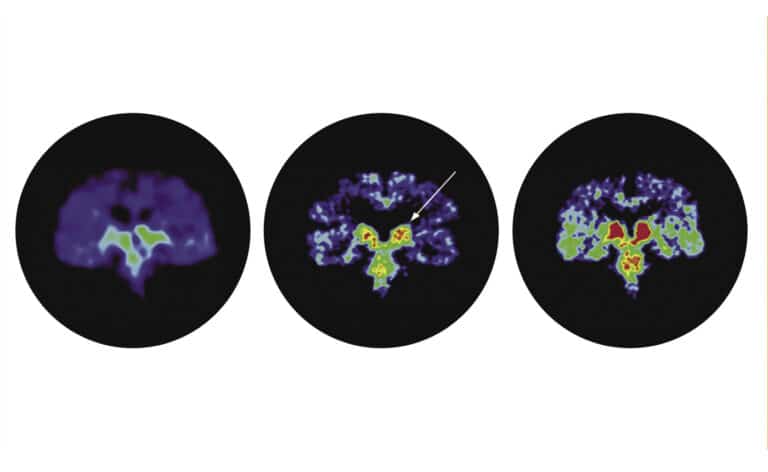
Studies that use a special magnetic resonance imaging method, called diffusion tensor imaging, to identify abnormalities in the brain are also underway. “The imaging looks at the perforant pathway, a particular collection of nerve fibers in the brain that connect the memory banks, the hippocampus, with other areas of the brain,” Mastrianni explains. “There are measures we can make that will let us know whether the neurons (brain cells) in that region are functioning poorly, which leads to a condition described in recent years as mild cognitive impairment (MCI). These [patients] are people who have some cognitive difficulty, which occurs with dementia, but not enough to impact their normal routine or alter their daily functioning. We’re trying to use this imaging tool to identify those who are at greatest risk for converting to Alzheimer’s disease, because they are the ones we want to target as early as possible with drug therapies that inhibit the progression of the disease, once they become available.”
The University of Chicago Medical Center is currently participating in a clinical trial that uses an antibody that attacks the bad proteins deposited in the brains of people with Alzheimer’s disease. “The antibody draws the damaging protein out of the brain and, hopefully, will prevent it from damaging additional brain cells,” Mastrianni says. “Within the next year to 18 months, we may have news on whether or not it’s working.”
While John Combs’ memory loss and cognitive abilities have continued to decline since his initial diagnosis, Mastrianni sees reasons to expect further advances on the study of memory loss as a whole. “I’m very optimistic on several fronts,” he says. “So much work has been done in the past several years on Alzheimer’s disease, with a focus on care and management and especially early detection… that we now have studies that indicate a preclinical phase of Alzheimer’s disease can be detected before any symptoms are visible. This will be extremely important for identifying “at risk” individuals before the disease takes hold, when therapies will be most effective.
Another positive signal is the intense research going on in the pharmaceutical industry; they recognize that finding a medication for Alzheimer’s disease will be a very profitable venture.
Published in Chicago Health Winter 2012

Nancy Maes, who studied and worked in France for 10 years, writes about health, cultural events, food and the healing power of the arts.