New therapies revolutionize sickle cell treatment, but who can afford the price tag?
Fact checked by Shannon Sparks
When the U.S. Food and Drug Administration (FDA) approved two milestone treatments for sickle cell disease at the end of 2023, the community rejoiced.
As a potential one-time therapy, the treatments might put an end to the patchwork of treatments people with sickle cell disease currently rely on: portable oxygen tanks, blood transfusions, opioids, and antibiotics, for example. This stew of therapies has become the standard of care during the 11 decades since a Chicago physician first identified and described sickle cell disease in 1910.
Now the U.S. counts more than 100,000 people diagnosed with this extremely painful condition, which some patients say feels like getting stabbed over and over again.
But along with the approvals came the therapies’ price tags: $2 million to $3 million. And based on these prices over a lifetime, medical experts already have resolved that most patients won’t have access to them.
“It’s one thing to approve them, but we need to make sure that patients have access to those therapies.”
Nicole Verdun, MD, recently took charge of the Office of Therapeutic Products within the FDA’s Center for Biologics Evaluation and Research. “The overall goal is to really get treatments that are safe and effective to patients quicker,” she says.
A graduate of the University of Chicago Pritzker School of Medicine, Verdun spent the first three years of her career as a resident at the Ann & Robert H. Lurie Children’s Hospital of Chicago. She says her switch from seeing patients to overseeing drug approvals at the FDA reflects her personal mission. “In the office that I’m leading, we have the unique ability to deliver very targeted and effective treatments for people with rare and life-threatening diseases, such as sickle cell disease.”
Approving the treatments was one thing. How the treatments will get to the patients who need them remains to be seen.
Medical mystery
The FDA’s approval of the therapies marked a new chapter in a medical mystery that began more than 100 years ago, when 20-year-old Walter Clement Noel went to Chicago Presbyterian Hospital seeking relief from a cough and a fever. He had moved to Chicago from Grenada, in the West Indies, to study dentistry at the Chicago College of Dental Surgery.
Physician James B. Herrick, MD, assigned Noel’s case to his intern, Ernest E. Irons, MD. Upon studying Noel’s bloodwork, Irons saw cells like none he’d ever seen before. Instead of smooth and round, the cells were shaped like crescents, or sickles.
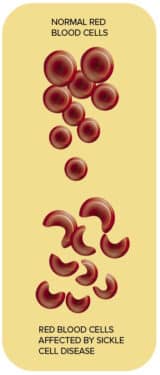
In people with sickle cell disease, a gene mutation creates hard and sticky sickle-shaped red blood cells. They accumulate in blood vessels, causing pain, strokes, and organ damage.
The sickle cells also die earlier than standard red blood cells, creating a red blood cell shortage.
Irons cared for Noel over the next two and half years until Noel returned to Grenada. There, he worked as a dentist before succumbing to pneumonia following a respiratory infection in 1916. He was 32.
People with sickle cell have a shorter life expectancy than people without the disease — two decades shorter, in fact.
While some individual states began screening earlier, U.S. hospitals didn’t begin universal screening for sickle cell disease at birth until 2006, despite the condition’s complications. Each year, about 300,000 infants are diagnosed with the disease.
In the U.S., sickle cell disease primarily affects Black people: 1 in 365. Around the world, it affects people in South America, the Caribbean, and Central America; Saudi Arabia; India; Mediterranean countries such as Turkey, Greece, and Italy; and people whose ancestors came from sub-Saharan Africa. Although some therapies exist to manage the disease, research into cures was stymied by limited funding, lack of awareness, and, some say, by racism.
Two medications offer hope for a cure
The two most recent FDA-approved sickle cell therapies offer hope to millions. And they work in very different ways.
Lyfgenia, a gene therapy developed by Massachusetts-based bluebird bio, Inc., harvests a person’s blood stem cells and uses a virus to genetically modify the cells to produce hemoglobin that has a lower risk of sickling. The modified stem cells are returned to the patient.
This therapy has been approved to treat patients 12 and older who have a history of vaso-occlusive events — episodes where the person’s tissues lose oxygen because the sickled red blood cells block blood flow.
According to bluebird bio Inc., 94% of patients who received the therapy have shown complete resolution of their vaso-occulusive events.
The other therapy, Casgevy, developed by Massachusetts-based Vertex Pharmaceuticals, Inc., uses genome editing technology to modify a patient’s harvested blood stem cells. Scientists edit the DNA sequence that creates the sickle blood cells and alter it with a sequence that leads to the production of red blood cells that do not sickle.
The modified blood stem cells are then transplanted back into the patient, where they attach and multiply within the patient’s bone marrow. This therapy has also been approved to treat patients age 12 and older with recurrent vaso-occlusive crises.
In Chicago, Northwestern Memorial Hospital is an approved treatment center for therapy with Lyfgenia. The University of Chicago Medicine and Comer Children’s Hospital is an approved treatment location for Casgevy, under the supervision of James LaBelle, MD, PhD.
“The wonderful thing about gene therapy the way it’s done now is that you use the patient’s own stem cells,” LaBelle says. “We don’t have to worry about many of the complications that can happen after a traditional sort of transplant where patients receive stem cells from either a related or unrelated donor.”
UChicago’s gene trial had three subjects. The first was Lyric Porter. A 2019 graduate of Florida Agricultural & Mechanical University, Porter blogs about her experience with sickle cell disease for WebMD.
Porter was diagnosed as a baby. “As a kid I actually had to take the oxygen tank to kindergarten,” Porter says. “My teacher would try to make it interactive and get people to like, help me drag the machine.”
As she got older, her pain crises would show up at the most inopportune moments.
“I agreed to go to another school’s senior prom, and I actually got sick the night before the prom. But I didn’t want to cancel on the prom. So I ended up going to the prom, but I was just in a lot of pain the entire time. It wasn’t as fun.”
Porter ended up in the emergency room the next day and was hospitalized after that. She had been in testing for a haploidentical stem cell transplant at UI Health when her hematologist at UChicago Medicine informed her about a gene therapy clinical trial opening at UChicago.
Since Porter underwent the therapy in 2022, her symptoms have improved.
“I still have some pain, but it hasn’t been as severe, and it hasn’t been as long- lasting as it was prior to the treatment,” Porter says, adding that she hasn’t had any more blood transfusions since the therapy.
Still, it’s unclear the extent to which private insurers, Medicare, and Medicaid will cover the cost of the treatment for the roughly 3,500 people in Illinois who have sickle cell disease.
Upon its approval, bluebird bio Inc., announced a price of $3.1 million for Lyfgenia, which the company says reflects the cost of current standard treatment over a patient’s lifetime, which may include frequent blood transfusions and prescriptions.
The pharmaceutical company says it “is in advanced discussions with the nation’s largest commercial payers and more than 15 Medicaid agencies representing 80% of individuals with sickle cell disease in the U.S.” The company says that it anticipates starting the treatment in 85 to 105 patients in 2024.
At a healthcare conference in January 2024, Vertex, maker of Casgevy, told an audience of healthcare stakeholders that it had started an agreement with Synergy, a healthcare payer covering approximately 100 million people.
Organizations like the Sickle Cell Disease Association of America also meet with Medicaid to discuss coverage of therapies.
“It’s one thing to approve them, but we need to make sure that patients have access to those therapies,” Verdun says. “And so that really is more of a partnership between the community, the companies, the Centers for Medicare and Medicaid Services, and others to make sure that that happens.”
Originally published in the Spring/Summer 2024 print issue.

A native of Chicago’s Marquette Park neighborhood on the southwest side, Cassie M. Chew operates as a freelance healthcare policy reporter based in D.C.