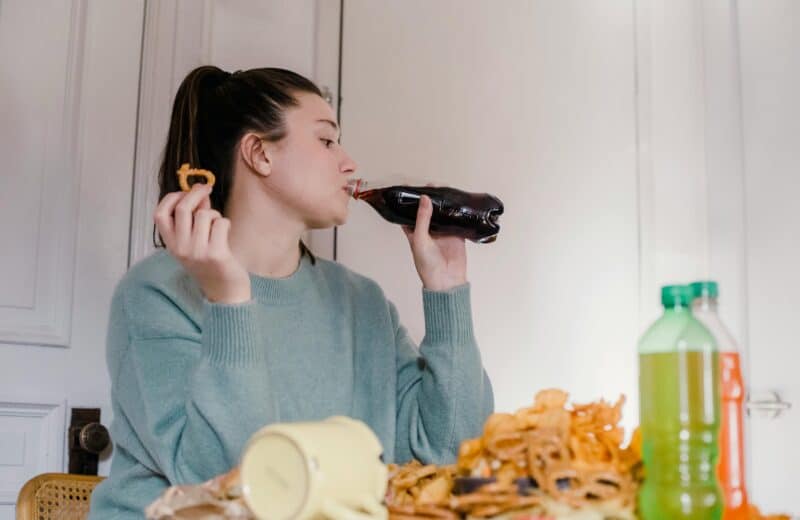Lee Miller, PhD, in his lab at Northwestern University. Photo by James Foster
Futuristic is the first word that comes to mind when learning about today’s leading-edge research on prosthetics. In Chicago, researchers and specialists are working on mind-boggling projects. Thanks to the Rehabilitation Institute of Chicago, patients who lost an arm can now move their prosthetic arms intuitively. At Northwestern University, researchers are developing methods to restore movement to people with spinal cord injuries using a brain/computer interface. And scientists at University of Chicago are creating prosthetic hands that can actually feel.
Here are some highlights of the prosthetics work—in practice and research—that are happening around Chicago.
Rehabilitation Institute of Chicago
When Walter Afable, CP, director of Specialized Services at the Rehabilitation Institute of Chicago (RIC), is asked about his most memorable patient, he recalls an electrical line worker from Boston who lost both his arms when an electrical current ripped through his body while on the job.
Unsatisfied by his limited progress and after a number of grueling surgeries, the man traveled to Chicago to pursue more advanced prosthetic devices. He chose to have his chest reenervated through targeted muscle reenervation, a surgical procedure developed by RIC and Northwestern. A surgeon took nerve endings that once controlled the patient’s arms and reassigned them to his chest, so that when he thinks about moving his arms, his prosthetic arms respond.
Afable explains, “When the relocated nerves activate, new patterns of electrical activity are generated in the muscles. The prosthetic arms decode these patterns and respond to the user’s intent in a more natural and intuitive way.”
The key to prosthetics today and in the future is to make them as intuitive as possible, Afable says. “Upper limb prostheses have a high rejection rate because they are cumbersome, heavy, uncomfortable to wear and lacking in intuitive control. We want our user just to think, ‘Open my hand,’ and the hand opens, as opposed to saying, ‘I’d really like to open my hand to grab this coffee cup. What do I have to do again? Oh right, that’s in my chest; I’ve got to move this muscle.’ Our belief is, if we can get closer to making truly intuitive devices, then the user’s willingness-to-accept rate will be much higher.”
Northwestern University
Lee Miller, professor in the departments of Physiology/Physical Medicine and Rehabilitation at Northwestern University Feinberg School of Medicine, has been studying the ways monkeys’ brains work when they’re using their arms and hands to perform certain tasks like grasping a ball. One ultimate goal of his lab’s work is to use that brain-activity pattern to allow people with spinal cord injury paralysis to use their limbs with the help of a neuroprosthesis—or a brain/computer interface.
It works like this: In a patient with a spinal cord injury, information from the brain isn’t able to get to the nerves of the arms to cause movement. It’s blocked. Scientists at Miller’s lab have found a workaround. Functional Electrical Stimulation (FES) uses electrical current to cause paralyzed muscles to contract. The trick, in a paralyzed patient, is to come up with a way to control those contractions. Using signals recoded from the brain might be that trick.
Northwestern researchers give a monkey a local anesthetic to cause temporary arm paralysis. They then give the monkey a ball to pick up. The monkey’s brain—which is implanted with an array of microelectrodes that can record the activity of individual brain cells—communicates that it is trying to pick up a ball. In doing so, the neuroprosthesis sends an electrical current to the paralyzed muscles, causing them to move as the brain is trying to tell them. In the lab, through FES, the monkeys can use their hands to pick up the ball, almost as they did before the paralysis.
“Because we can look at the monkey’s brain and figure out what he’s trying to do with his muscles, we can electrically stimulate them, cause them to contract artificially and essentially restore the monkey’s voluntary control. The signals go to the paralyzed muscles through our computer and electrical stimulator instead of through the spinal cord,” Miller says.
One particular challenge is in developing the FES part of the neuroprosthesis so that it can cause the complex hand movements we are naturally capable of making. Another challenge facing all brain/computer interfaces is that the array implanted in the brain only works, on average, for one to two years and has the potential to damage the brain.
Scientists, therefore, must develop a more biocompatible method before it can move beyond the lab.
University of Chicago
Sliman Bensmaia, PhD, associate professor in the Department of Organismal Biology and Anatomy at The University of Chicago, is tackling a significant challenge in the realm of prosthetics: feeling. Without sensory signals, prosthetic devices feel awkward, clumsy, slow. Bensmaia is working to change that.
The ability to feel relies on the sensory parts of the brain. For people who have lost an arm or hand, the part of the brain that communicates touch in those areas is dormant. In his lab work with monkeys, Bensmaia electrically stimulates the sensory parts of the brain through implanted electrodes to elicit touch sensations. He’s trained monkeys to recognize (nonpainful) pokes on their hand and has shown that if he replaces those pokes with electrical stimulations to the brain, the monkeys react as if they had been poked in a specific way, implying that they experience the electrical stimulation of the brain as touch sensations.
The long-term goal: When a prosthetic device touches or is touched, the person will feel that sensation because the brain will be electrically stimulated. “Any time the index fingertip touches something, we will inject some current through an electrode in the brain that used to receive sensory input from the finger. What we’ve shown is when you do that, the patient will feel something on his or her index fingertip,” Bensmaia explains.
Challenge: The electrodes that are implanted in the brain only work for a few years and cannot be removed or replaced. Scientists must develop a method that is viable in the longer term before it can move beyond the lab.