Immunotherapy treatments rev up immune system to fight cancer
Six years ago Henry Kawell, then 72, heard news that rerouted his life: He had a tumor growing in his right lung.
His oncologist ordered the tumor removed via surgery, but there was more cancer in the lining of Kawell’s lung. He underwent two years of chemotherapy and could only stay on the first drug for six months before switching. “It was just so powerful. I could hardly use my right arm for a while,” he says.
The chemotherapy also sapped Kawell’s energy, but scans showed no cancer activity—until 2013. Then five tumors appeared in Kawell’s right lung, each less than an inch long. Oncologist Maria Quejada, MD, at Edward-Elmhurst Health, recommended more chemo. Kawell prepared for another battle.
Because cancer cells are particularly adept opponents, they can shield themselves from the immune system, growing fast and spreading far. At the University of Chicago Medicine, medical oncologist Jason Luke, MD, FACP, explains that some cancers block the immune system from recognizing cancer cells or block the immune system from attacking.
The major issue researchers face now is why the immune system sees and acts on some cancers but not others. “That’s the 50-zillion-dollar question,” Luke says.
Oncologists hope the answer lies in teaching patients’ immune systems to recognize and fight cancer. Enter their latest weapon, full of promise: immunotherapy.
“Immunotherapy has revolutionized our approach to cancer treatment,” Luke says. “This will be the center of cancer research over the next decade.”
Looking Back
Immunotherapy is an umbrella term. Some immunotherapies target immune cells against cancer cells that carry specific antigens. Others use immune cells to attach toxins to the surface of cancer cells. And others stimulate immune cells into battle, says Leonard Klein, MD, a hematologist and medical oncologist with Illinois Cancer Specialists.
Scientists began looking to the immune system to fight cancer in the 1960s and ’70s. They noticed that some patients went into spontaneous remission if they developed an infection. Perhaps the infection had jump-started the immune system. To test that theory oncologists injected BCG, a vaccine created from tuberculosis, into the bladder of some patients with bladder cancer.
It worked. Over the next few decades, scientists developed other immunotherapies that focused on proteins—interleukin and interferon—that modulate the immune system.
In the Lab
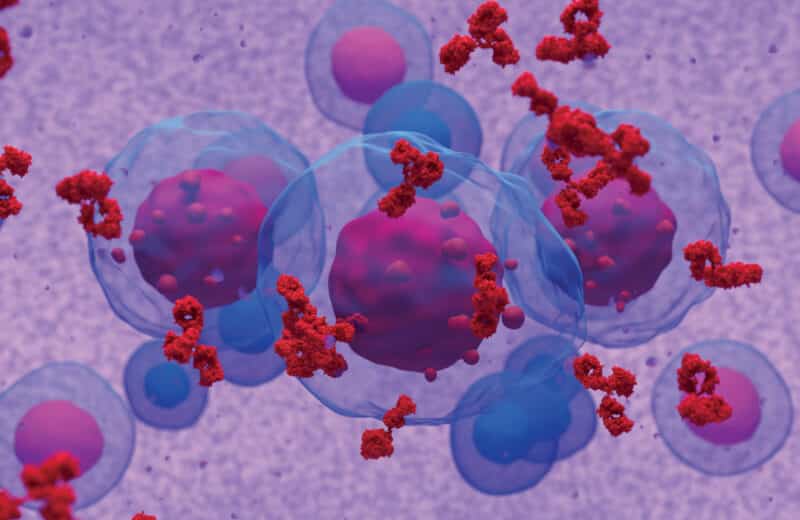
Today, researchers are focusing on the series of checks and balances that tells the immune system to attack something abnormal in the body. Some cancers take advantage of those checks and balances, tricking the checkpoints into thinking cancer cells aren’t foreign.
“They put their hand up and block the cells of our immune system from killing them off,” Klein says.
The latest form of immunotherapy treatments, called checkpoint inhibitors, stop cancer cells from blocking the immune system.
“This is one of the newest and most important steps coming right from the lab to the bedside. It’s moving very, very quickly,” Klein says.
But revving up the immune system doesn’t always work. In some people, a hyperactive immune system can attack the body’s organs, so physicians monitor closely and use treatments such as steroids to counteract side effects.
“Just like every other drug we give in the body, there are consequences. Nothing is a free ride,” Luke says.
On the Fast Track
The Food and Drug Administration (FDA) already has approved checkpoint inhibitors for multiple cancers:
Ipilimumab (Yervoy)—FDA-approved for late-stage melanoma in 2011
Nivolumab (Opdivo)—FDA-approved for advanced melanoma in 2014; non-small cell lung cancer and renal cell carcinoma in 2015; classical Hodgkin lymphoma in 2016
Pembrolizumab (Keytruda)—FDA-approved for advanced melanoma in 2014; non-small cell lung cancer in 2015
Atezolizumab (Tecentriq)—FDA-approved for urothelial carcinoma bladder cancer in 2016
Matthew Siegel, MD, a hematologist and oncologist with Edward-Elmhurst Health, says these immunotherapy treatments can extend life for patients with metastatic, incurable cancers. “Standard chemotherapy works for a period of time, but then stops working. We’re seeing some people respond [to immunotherapy] for a really long time,” he says.
Pembrolizumab made big news in 2015 when former President Jimmy Carter announced he had no detectable melanoma after taking the drug (combined with surgery and radiation) for roughly four months. He had been diagnosed with melanoma in his liver and brain.
The FDA has fast-tracked the therapies for additional cancers, including Merkel cell carcinoma. Head and neck cancer, triple negative breast cancer, glioblastoma and mesothelioma aren’t far down the line.
But while there’s plenty of promise, oncologists refrain from calling anyone “cured.”
“Patients who would have died within three to six months are now living years. Every time things work, they may not work 100 percent, but it gives us clues,” Klein says.
Klein adds that he has seen “wonderful things on the horizon that tend to go away. People who have been in the field for a long time understand: It’s not the final answer, but it’s a building block.”
Luke echoes Klein’s reaction. “With cancer, we’ll only know you’re really cured if you eventually die of something else. We’re slowly making progress at long-term survival rates, but we’re just at the beginning of understanding this.”
A Promising Future
Oncologists currently use immunotherapy as a second or third line of defense. “While immunotherapies continue to show promise as new options for many cancers,” Siegel says, “in some cases when effects are not yet well understood, it may be better to exhaust other known therapies before they are given.”
That’s exactly when Kawell’s oncologist introduced the idea to him—when his tumors grew despite chemotherapy. Since November, he has visited Edward Hospital every two weeks for an injection of nivolumab. His only side effect has been a slight lack of energy. It’s nothing compared to the rigors of chemotherapy, he says.
In January 2016, scans showed that Kawell’s two larger tumors had shrunk by 30 percent while the others remained the same size. Then in May 2016, scans showed that the three largest tumors had disappeared altogether, and the two remaining tumors were so small they were declared “unspecified”—too small for the doctor to determine whether they were cancer anymore.
For Siegel, Kawell’s results are exciting. “You know what trials have shown, and you have your expectations, but sometimes you’re blown away.”
Luke encourages patients to seek opportunities and to look for trials on ClinicalTrials.gov. “Take ownership. Push the envelope. Ask your doctors. Look for support groups,” he says.
Klein says, “Cancer is not hopeless. The speed of access to newer trials for most patients is increasing and helping all cancer patients.”